Introduction
Wearable physical activity trackers have become increasingly common within clinical studies, patient monitoring, and health assessments. Physical activity is a key endpoint of interest when evaluating treatments for hemophilia. The objective of this analysis was to describe the psychometric properties of five outcome measures of physical activity from the accelerometer activity monitor used in the XTEND-1 study.
Methods
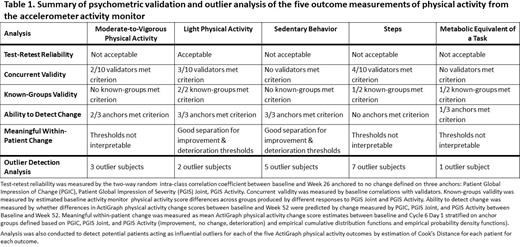
XTEND-1 (NCT04161495) was a Phase 3, open-label, multicenter study of the efficacy, safety, and pharmacokinetics of efanesoctocog alfa in previously treated adults and adolescents aged ≥12 years with severe hemophilia A. Patients receiving prior standard-of-care prophylaxis factor VIII (FVIII) received once-weekly (qw) prophylactic efanesoctocog alfa for 52 weeks (Arm A); patients on prior on-demand FVIII treatment received efanesoctocog alfa on-demand for 26 weeks, then prophylactic efanesoctocog alfa qw for 26 weeks (Arm B). Patients wore a triaxial medical grade accelerometer activity monitor device for continuous monitoring during pre-specified intervals 8 consecutive days after (or before) scheduled visits (to collect data for a full 7-day week + 1 additional day): after the screening, baseline, Week 4, 13, 26, 39 visits, and before the Week 52 visit. Compliance was assessed based on number of monitoring intervals and number of days wearing the device, and number of minutes spent wearing the device within those days. Five outcomes were assessed: light physical activity (LPA), moderate-to-vigorous activity, sedentary behavior (minutes/day spent in these individual outcomes out of total number minutes wearing device while awake), steps (number of steps taken per day), and metabolic equivalent of a task (MET; total estimated metabolic rate per day). Psychometric analyses of these outcomes using data from Arm A were conducted to evaluate test-retest reliability (TRTR), concurrent validity, known-groups validity, ability to detect change, and meaningful within-patient change (MWPC) of physical activity measurements ( Table 1). An analysis to detect potential influential outlier patients for each outcome that could potentially bias results, was conducted.
Results
In total, 132 out of 133 patients in Arm A and 26 out of 26 patients in Arm B, distributed in 19 countries, had accelerometer data. Compliance was defined post-hoc as ≥12 consecutive wear hours/day and results are presented for days achieving this threshold; no meaningful differences in results were found when incorporating number of monitoring intervals or number of days within the interval in the compliance definition.
Most patients maintained or improved physical activity as measured by the activity monitors, with an overall increase in mean (SD) minutes during each monitoring period for LPA (baseline: 636 [234]; Week 52: 653 [237]), though there were variable changes throughout the study period. Measurements of LPA demonstrated acceptable TRTR, concurrent validity with three validation criteria, and ability to detect change with all or most of the anchor variables, while demonstrating good separation for improvement and deterioration thresholds to provide for MWPC ( Table 1). Two patients were identified as influential outliers for LPA. For moderate-to-vigorous activity, the concurrent validity showed that two validators met the criteria, and for steps, four validators met the criteria. Sedentary behavior and MET did not meet criterion with any of the validators ( Table 1). Out of the outcomes assessed, LPA showed the best psychometric properties within the study.
Conclusion
These analyses show how physical activity tracking through wearables was used to assess outcomes in patients with severe hemophilia A, who switched to a FVIII replacement therapy that provided normal to near-normal FVIII activity for most of the weekly dosing interval, resulting in reduced pain and improved physical functioning as assessed by patient-reported outcome measures. Analyses of these results revealed limitations in the psychometric properties of the assessed outcomes. To further understand the personal motivation and barriers for improving physical activity, addition of goal-setting could be considered in future clinical studies.
Disclosures
Wilson:Sanofi: Current Employment, Current equity holder in publicly-traded company. Msihid:Sanofi: Current Employment, Current equity holder in publicly-traded company. Ayasse:OPEN Health: Ended employment in the past 24 months. Dumont:Sanofi: Current Employment, Current equity holder in publicly-traded company. Willemze:Sanofi: Current Employment, Current equity holder in publicly-traded company.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal